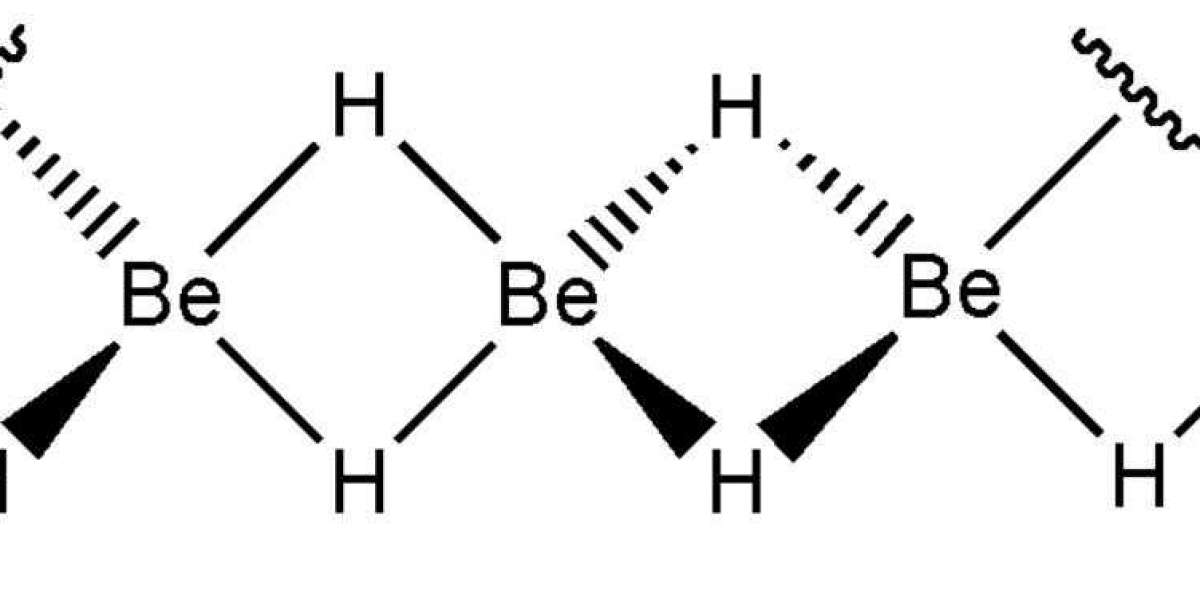
γ-BeH2, the configuration of α-BEH2 can Be viewed as a single layer of BE atoms sandwiched between two layers of H atoms. In this way, α-BEH2 can be thought of as distorted γ-BEH2. The bond Angle of H-BE-H increases from 90° in γ-BEH2 to 109.4° in α-BEH2, while the bond length of BE-H decreases slightly from 1.48 to 1.46 A, still longer than the bond length of 1.43 A in bulk BeH2. Thus, the structural distortion of α-BEH2 leads to higher stability. In β-BEH2, each Be atom is hexagonally coordinated with six neighboring H atoms, each H atom bonded to three Be atoms on either side of the plane in an alternating fashion. Interestingly, the bond length of BE-H in β-BEH2 is calculated to Be 1.59 A, much longer than (1.43 A) in the ontology BeH2 at the same theoretical level. To our knowledge, this is the longest Be-H bond length reported to date.
So why are α-BEH2 and β-BEH2 structures more stable than γ-BEH2? Even the previous structure had longer Be-H bond lengths? To understand this, we evaluated the cohesion energy to obtain the experimental feasibility of these two predicted BeH2 monolayers, defined as Ec= (nBeEBe+nHEH−EBeH2)/(nBe+nH). Where EBeH2 is the energy of alpha - or beta - BeH2. EBe and EH are the energies of individual Be and H atoms. In this definition, the larger the Ec, the higher the stability. According to our calculations, the Ec values of α-BEH2 and β-BEH2 are similar, up to 2.76 eV per atom, 0.14 eV smaller than the bulk BeH2 (2.90 eV per atom), and higher than the experimentally synthesized nanocluster BenH2n (n= 3-9) (2.54-2.71 eV per atom). Much higher than the 2.34 eV per atom Ec in γ-BEH2. For comparison with other popular 2D materials, using the same theoretical approach, the cohesion energies of graphene, Cu2Si monolayers and germanene are 7.85, 3.46 and 3.26eV/ atom, respectively. Therefore, both α - and β-BEH2 have high structural stability.